Abstract
Introduction: B-cell maturation antigen (BCMA)-targeting immunotherapies (eg, chimeric antigen receptor T cells (CAR-T) and bispecific antibodies (BsAb)) have achieved remarkable clinical responses in patients (pts) with relapsed and/or refractory multiple myeloma (RRMM). Their use is accompanied by exaggerated immune responses related to T cell activation and cytokine elevations leading to cytokine release syndrome (CRS), which could be life-threatening if presenting in high grades. This study utilized a meta-analysis approach to compare CRS profile in BCMA-targeting CAR-Ts vs. BsAbs in pts with RRMM.
Methods: CRS profiles for BCMA-targeting CAR-T and BsAb therapies were compiled from publicly available data in published literature up to July 2022. The meta-analysis included 28 CAR-Ts, including 2 approved therapies (idecabtagene vicleucel and ciltacabtagene autoleucel) and 8 investigational BsAb. The weighted proportion of all Grades, Grade 1-2, and Grade ≥3 CRS was estimated using fixed- and random-effects meta-analytic models. Between-study heterogeneity was evaluated using the heterogeneity index (I2) and P-value. Subgroup analyses were performed to assess the differences in CRS incidence and severity across CAR-T and BsAb and routes of BsAb administration (IV vs. SC). Dosing, priming/premedication (premeds) regimens, time to onset, duration of CRS, and CRS management were compared.
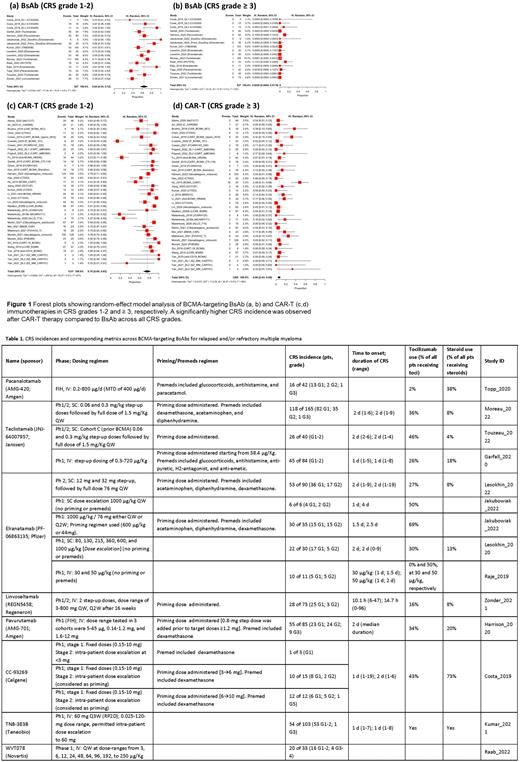
Results: A total of 53 studies including 2092 RRMM pts treated with BCMA-targeting CAR-T and BsAb therapies were included in the analysis. The meta-analysis showed a significantly higher weighted proportion of CRS with CAR-T therapies vs. BsAb (all Grades 87% (95% CI: 80-93) vs. 67% (95% CI: 58-75), I2= 87%, P-value <0.01; and Grade ≥3 6% (95% CI: 3-9) vs. 0.2% (95% CI: 0.0-1.7), I2= 65%; P-value <0.01 (Figure 1). Average time to CRS onset was delayed (5 days vs. 1 day) and the median duration of CRS was longer (5 days vs. 2 days) after CAR-T compared to BsAb. Use of premeds (eg, corticosteroids, antihistamines, and antipyretics) was reported for 5 BsAb agents, while premeds were not used to reduce CRS severity with CAR-Ts. Tocilizumab and corticosteroids were used to manage CRS in 50% (325/649 pts; 12 studies) and 17% (87/525 pts; 9 studies) of patients, respectively, treated with CAR-T therapies, as compared to 31% (212/677 pts; 13 studies) and 16% (102/625 pts; 9 studies) of patients, respectively, treated with BsAb agents. Two BsAbs evaluated both IV and SC routes (Table 1). A higher incidence of CRS Grade 1-2 was found following SC compared to IV route of BsAb administration: 73% (95% CI: 62-83) vs. 58% (95% CI: 48-68) (I2=73%; P-value= 0.05), respectively. However, higher doses were evaluated with SC compared to IV routes. A higher incidence of CRS Grade ≥3 was observed with BsAb administered IV vs. SC: 0.9% (95% CI: 0.0-4%) vs. 0.0% (95% CI: 0.0-0.3%), respectively (I2= 49%; P-value = 0.05). CRS occurrence by dose number administered was reported for 3 BsAbs. Except for teclistamab, which reported CRS events in 4.8%, 2.4%, 1.2% and 3.6% of pts following the 4th, 5th, 6th, and later doses, respectively, the other 2 BsAbs (CC- 93269 and elranatamab) reported CRS episodes mostly following either the first or second dose, and rarely with the third or later doses.
Conclusions: The current analysis showed BCMA CAR-T therapies to be associated with higher CRS incidence, higher rates of Grade ≥3 CRS, longer CRS duration, and more prevalent tocilizumab and steroid use for CRS management compared to BsAb, possibly due to a) higher cytokine production following CAR-T therapies and/or b) use of premedication and priming regimens with BsAb which may improve the CRS profile (Table 1). Despite evaluation of a higher dose range, the proportion of CRS Grades ≥3 was higher with BsAb dosed IV vs. SC suggesting that the SC route of administration may allow exploration of higher doses while reducing the incidence of high-grade CRS. This meta-analysis showed that different types of BCMA-targeting immunotherapies and administration routes could contribute to different CRS profiles. The analysis is limited to published literature, and biases due to missing data or different reporting approaches cannot be excluded (eg, several BsAbs reported CRS rates pooled across doses). The analysis will be updated upon availability of full publications with more granular data.
Disclosures
Sharma:Pfizer: Ended employment in the past 24 months. Wang:Pfizer: Current Employment. Lon:Pfizer: Current Employment. Soltantabar:Pfizer: Current Employment. Viqueira:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Czibere:Pfizer: Current Employment. Hickman:Pfizer: Current Employment. White:Pfizer: Current Employment, Current equity holder in publicly-traded company. Elmeliegy:Pfizer: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.